
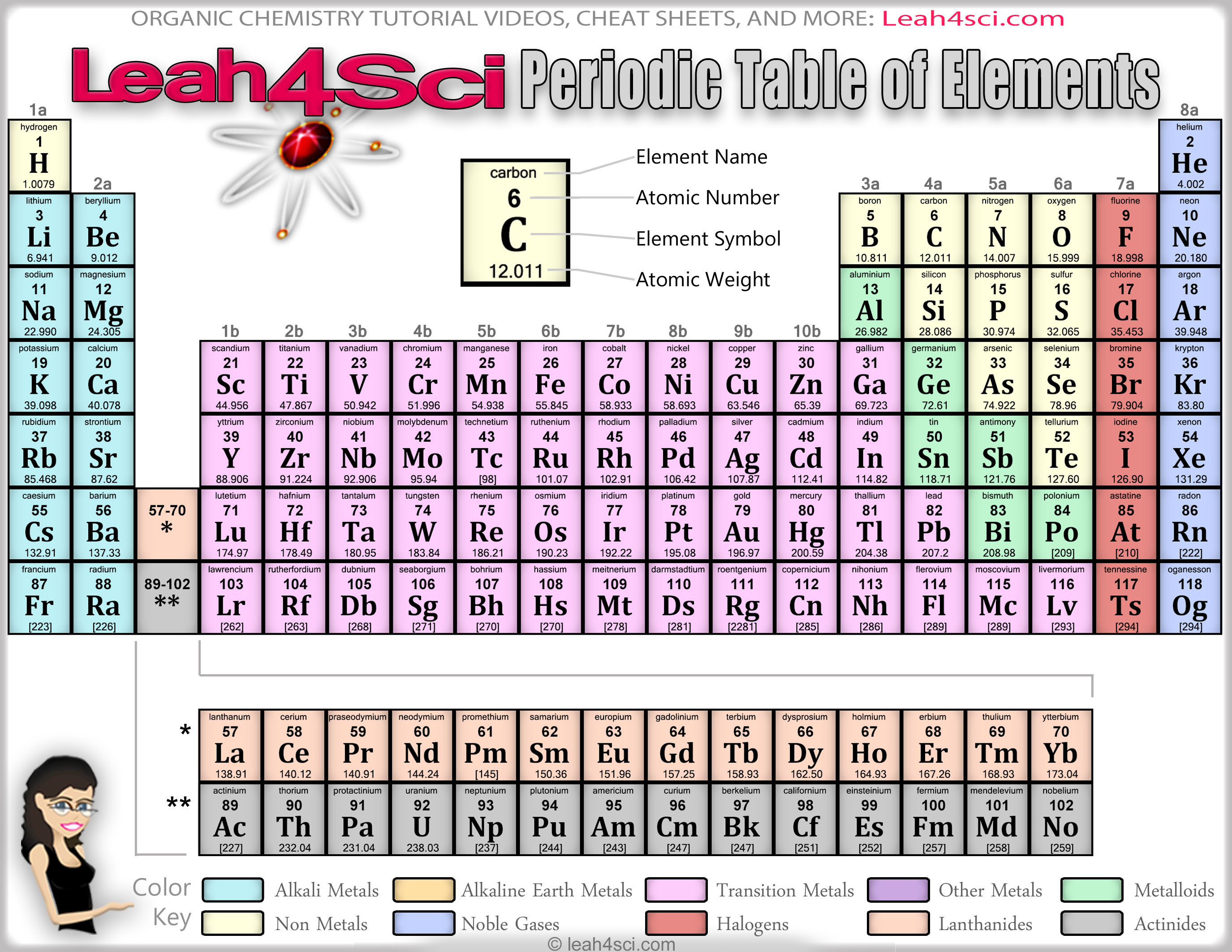
Alkali Metals: These metals do not occur freely in nature because they react violently with water.The ADDucation Period Table Groups (aka Classifications) are: chemical elements that share similar properties are organized in columns. You will find chemical elements grouped, spelt and colored differently in various versions of the periodic table but the underlying principle remains the same i.e. The symbol W for Tungsten refers to Wolfram the mineral found in wolframite, from the German wolf rahm/ wolf’s foam – the amount of tin consumed to extract Tungsten. The symbol Sn for Tin is taken from latin stannum probably derived from the Indo-European word stag (dripping) because tin is easily melted. The symbol Na for Sodium is taken from Latin natrium, Greek nítron and earlier Arabic natrun. Argentina is the only country named after a chemical element. The symbol Ag for Silver is taken from Latin argentum probably derived from an Indo-European word for shiny metal. The symbol K for Potassium is taken from Latin Kalium. Alchemists believed hydrargyrum was close to gold so named it after the planet closest to the Sun, which is Mercury. The symbol Hg for Mercury is taken from Greek hydrargyros (liquid silver or quicksilver in English) resulting in hydrargyrum. The symbol Pb for Lead is taken from Latin plumbum probably derived from an earlier language than Greek. The symbol Fe for Iron is taken from Latin errum which means iron or sword. The symbol Au for Gold is taken from Latin aurum (yellow) and from aurora (dawn). The symbol Cu for Copper is taken from Latin Cuprum a contraction of Cyprian metal from Cyprus which was famous for copper. The symbol Sb for Antimony is taken from Latin stibium, Greek stíbi which means eye paint because antimony was used in eye cosmetics. Here’s a list of chemical elements with symbols which do NOT match their names with explanations: Most of the abbreviations for elements are derived from Latin. Why do some elements have names which do not match its symbol? 18 columns: Columns are used to group together chemical elements which share similar properties.

#Printable periodic table of elements with names full#
The last element in each row has a full shell of electrons so these elements are the least reactive and most stable. The first element in each row has just one electron in its outer shell which means it is unstable and the most reactive. All the elements in the second row (second period) have two orbitals for their electrons, as so on for the other periods. For example all the elements in the top row (period) has one orbital of its electrons.

All of the elements in the second row (the second period) have two orbitals for their electrons.Ĭhemical elements are grouped into periods (rows), ordered by their atomic number, which have the same number of atomic orbitals. For example, every element in the top row (the first period) has one orbital for its electrons. What does “Periodic” mean in the periodic table?Įlements in a period have the same number of atomic orbitals. Frequently Asked Questions About the Periodic Table.


 0 kommentar(er)
0 kommentar(er)
